|
Regarding the magnetic properties of FH, the main results point to a cluster-like structure with Fe3+ ions antiferromagnetically ordered within the core and a small uncompensated magnetic momento (probably at the particle surface). But the actual physical units from which the observed cluster or superparamagnetic (SPM) behavior is originated have not been univocally defined. For antiferromagnetic (AFM) particles of few-nanometer diameter, a small uncompensated magnetic moment is expected to arise from defects within the particle and/or from the unpaired surface moments of the particle. The net magnetic moment of the particle reverses among different spatial orientations with a characteristic time τ that depends on temperature and particle volume. |


|
Spinel Oxides (a.k.a Ferrites) |

|
Ferrites are most common in nature. From the daily iron oxide Fe3O4 (magnetite) formed at the surface of many iron-containning objects (fences, cars, doors, barbecue grills, etc), we witness the effect of the atmosphere's oxygen acting on the surface of metal. These materials are quite simple from the structural point of view. However, their magnetic properties can greatly vary from one element to another, since the microscopic (atomic) structure is composed of two or more magnetic sub-units (sublattices). |
|
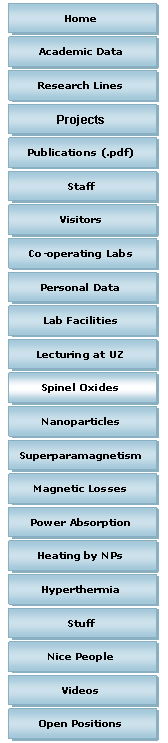
The ionic distribution in this kind of structure may be represented by [MdFe1-d]A[M1-dFe1+d]BO4, where d is the inversion parameter and d=0 and 1 stand for the inverse and normal cases, respectively. Although most spinel ferrites are cubic, there are some exceptions like CuFe2O4 that can have tetragonal unit cell symmetry if the sample is slowly cooled from high temperatures. |
|
Further References: For a more advanced view, go to: · The Institut Laue-Langevin (ILL) Site |
|
The spinel ferrite structure MFe2O4 can be described as a cubic close-packed arrangement of oxygen ions, with M2+ and Fe3+ ions at two different crystallographic sites. These sites have tetrahedral and octahedral oxygen coordination (A- and B-sites respectively), so the resulting local symmetries of both sites are different.
|
|
Magnetite (Fe3O4) |
|
Ferrihydrite (Fe5HO8 · 4H2O) ) |


|
Ferrihydrite (FH) phase is found in many biological systems (e.g., iron-reducing bacteria, Aedes aegypti mosquito), as well as in hydrometallurgical operations (as an undesired precipitate). Along with the interest of the basic physical mechanisms governing structure and surface formation, FH has also significance because it constitutes the core of ferritin, a protein that plants and animals use to sequester and store iron, providing a fully biocompatible material to carry iron particles in potential drug delivery applications.
|
|
This text on FH was extracted from
“Large magnetic anisotropy in ferrihydrite nanoparticles synthesized from reverse micelles”, E L Duarte, R Itri, E Lima Jr, M S Baptista, T S Berquó and G F Goya, Nanotechnology 17 5549-5555 doi:10.1088/0957-484/17/22/004
|
|
Electron Microscopy and Mossbauer spectroscopy have been employed quite successfully for identifying the properties of both kind of structures regarding their local structural and magnetic properties. The current landscape is that some aggregates with ordered structure, with small regions of ca. 2-3 nm, display definite lattice fringes and narrow distribution of hyperfine parameters.
|
|
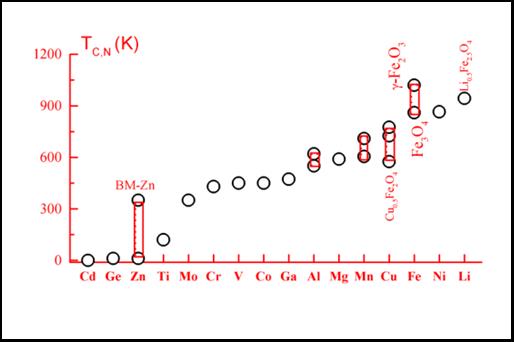
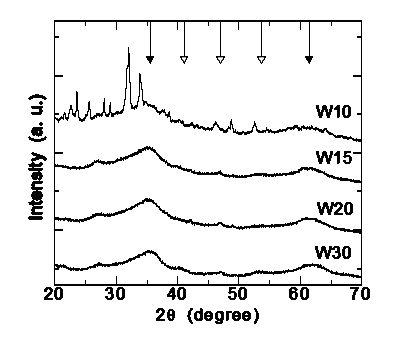
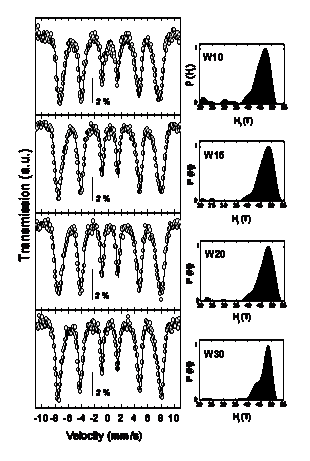
Ferrihydrite is a poorly crystalline Fe3+ oxyhydroxide whose detailed crystal structure has remained elusive up to now, as reflected by the identification of the known phases as '2-line' and '6-line' on the basis of their two or six broad X-ray diffraction (XRD) peaks, as shown in the figure on the rigth. It has been proposed that the 2-line type consists of extremely small crystalline domains, whereas the 6-line has larger crystal domains of hexagonal structure with unit-cell with parameters a = 2.96 Å, c = 9.40 Å. More recently, Jansen et al. have refined their XRD data by considering a space group P-31c phase plus a defective phase with P3 space group.
|
|
|
|
Hyperfine parameters of four ferrihydrite samples with increasing crystallite sizes from 2.8 to 4.0 nm |
|
Magnetite Fe3O4 has recently attracted attention because bulk Fe3O4 has a high Curie temperature (TC ~ 850 K) and nearly full spin polarization at room temperature, both properties of great potential for applications in giant magnetoelectronic and spin-valve devices based on magnetite films. In spite of the fact that magnetite is perhaps the oldest magnetic material known (see here and here), some aspects on the basic mechanisms related to the Verwey transition are still being discussed, especially in nanosized systems. An additional issue to be solved for potential applications is that the magnetic properties of magnetite-based nanostructured systems (particles or films) strongly depend on the synthesis route, as well as on the nonmagnetic matrix (or substrate) chosen. Moreover, for a given synthesis technique the final magnetic properties seem to depend heavily on tiny changes in the local structure like antiphase boundaries (APB), oxygen deficiency and local ionic disorder. |
|
Above the Verwey temperature, TV ~120 K, magnetite has a cubic spinel structure (space group Fd3m), with lattice parameter a0 ~ 8.397 Å and the O atoms arranged in an face-centered cubic (fcc) lattice. The lattice can accommodate Fe3+ on the tetrahedral site (A) and Fe3+ and Fe2+ on the octahedral site (B) in antiparallel arrangement, yielding ferrimagnetic order below TC. Bulk magnetite has cubic magnetic anisotropy, with the <1 1 1> and <1 0 0> directions being the easy and hard axes of magnetization, respectively. At room temperature (RT), the first-order magnetocrystalline anisotropy constant has a negative value (K1 = -1.35x105 erg/cm3) that changes sign at low temperature, passing through an isotropic point at a temperature few degrees above the Verwey transition. On cooling below TV, the change from cubic to triclinic structure yields a change to uniaxial anisotropy with <0 0 1> easy axis. Since the magnetic hardness of single-domain particles results from the concurrent effects of shape and crystal anisotropies, there is an obvious need for separating these effects to study each of these alone. |
|
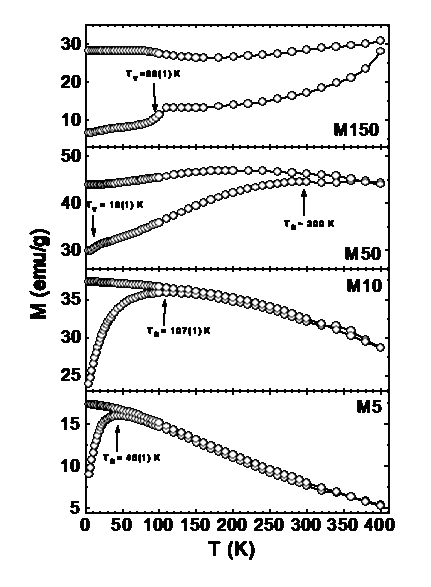
The saturation magnetization values found in nanostructured materials are usually smaller than the corresponding bulk phases, provided that no changes in ionic configurations occur. Accordingly, experimental values for MS in magnetite nanoparticles have been reported to span the 30-50 emu/g range, lower than the bulk magnetite value ~90 emu/g. Many studies have been reported on the origin of the observed reduction in magnetization in fine magnetic particles. The first studies on the decrease in magnetization performed in g-Fe2O3 by Coey showed that this reduction is due to the existence of noncollinear spins at the surface, making the same mechanism appealing for Fe3O4. |
|
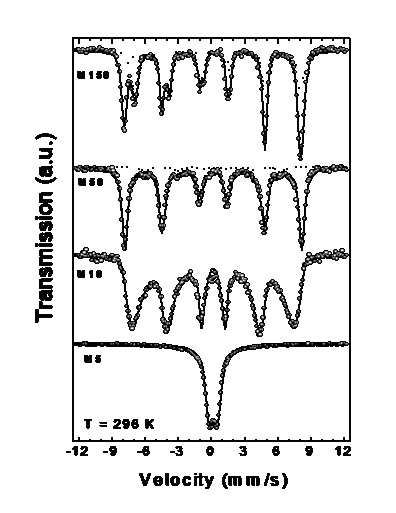
The Mössbauer spectra of Fe3O4 particles with different sizes, at 296 show the gradual passage from bulk-like to SPM behavior, as the average particle size decreases. For sample with <d> = 150, the RT spectrum displays two magnetic components of hyperfine fields Bhyp = 49.5 T and 46.0 T, corresponding to Fe3+ ions at sites A and (Fe2+ Fe3+) ions at site B, respectively with nearly null quadrupole splitting QS and intensity ratio B:A ~ 1:1. These well known features of MS are related to the electron transfer process (electron hopping) between Fe2+ and Fe3+ ions on the octahedral B site taking place for T > 115–120 K (Verwey transition). The absence of SPM signal (doublet) in the MS shows that these particles have a multidomain structure, in agreement with the theoretical estimation for maximum linear dimensions of single-domain particles of ~128 nm. As particle size decreases, both hyperfine fields merge into a single sextet (sample with <d> = 50 nm), and for sample with <d> = 10 nm the thermal relaxation effects are clearly observed. Sample with <d> 5 nm displays only a central doublet corresponding to a full superparamagnetic state. In this figure, the sample with <d> = 50 nm showed a small component from goethite (Bhyp = 37.7 T) amounting a ~3% of the total spectral area. |




|
MFe2O4 Structure |
|
Spinel ferrites and their related structures have been investigated for nearly one century, due to their theoretical and technological relevance. A great number of methods have been successfully employed to synthesize these magnetic ceramics with improved properties for specific applications, such as magnetic powders for massive storage devices.
|
|
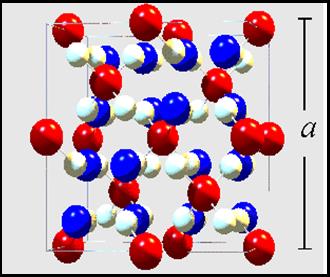
These materials are usually ferrimagnetic due to the strong A-A Ferromagnetic and A-B Antiferromagnetic coupling, showing Curie (Néel) temperatures spanning a borad temperature range, 3 K < TN,C < 1100 K, depending on the M cations, as shown in the figure on the right. |
|
Replacing one A-site Fe3+ ion with a B-site Cu2+ ion and vice-versa, results in a magnetic moment m=7´1mB + 2´5mB - 1mB =16mB, assuming that each Fe3+ ion contributes 5 mB and a Néel-type collinear spin structure. Thus a single Cu2+ ion per unit cell on an A site doubles the magnetic moment. However, it has been found that using the thermal quench method [6], the magnetic moment of CuFe2O4 cannot be increased much beyond 16mB per unit cell, since the activation energy of the process increases with the presence of one Cu2+ ion on an A site, thus making the transfer of a second Cu2+ ion very unlikely. |
|
Zinc ferrite (ZnFe2O4) has been a particular subject of study because of its contrasting magnetic properties compared to other spinel ferrites, e.g. low ordering temperature and antiferromagnetic ground state. This particular compound has a normal spinel structure, in which the iron atoms are located at B (octahedral) sites and Zn atoms occupy the tetrahedral A sites.(See Picture HERE) Whereas most iron-rich ferrites are ferrimagnetically ordered at room temperature, the antiferromagnetic transition temperature (TN) of stoichiometric, well-crystallized ZnFe2O4 is TN = 10±1 K. This difference is originated in the much weaker superexchange interactions between B sites than the corresponding A-B interactions. For this reason, a small migration of Fe atoms from B to A sites can originate ferrimagnetic regions with strong A-B superexchange interactions. In fact, this partial inversion has been frequently invoked as the source of the much larger TN observed in ZnFe2O4 fine particles, but it remains unclear whether the observed inversion is due to the chemical routes usually employed for nanoparticle synthesis, such as sol-gel and mechanical grinding, or is an intrinsic property of nanometric ZnFe2O4 particles due to finite-size effects. |
|
The ideal inverse configuration of Copper Ferrite (CuFe2O4) consists of 8 divalent (Cu2+) ions on the octahedral (B) sites and 16 trivalent (Fe3+) ions equally divided between the tetrahedral (A) and B sites per unit cell. The magnetization of sublattice A is antiparallel to that of sublattice B, whereas the magnetic moments of the ions on the A and B sublattices are ferromagnetically ordered. The total magnetic moment of CuFe2O4 is entirely due to the uncompensated magnetic moments of the eight Cu2+ ions on B sites. The magnetic moment per unit cell is m=8x1mB=8mB, assuming that each Cu2+ ion contributes 1 mB, where mB is the Bohr magneton. For MFe2O4 (M= metal element) spinel ferrites, the energy difference between Fe occupations in A and B sites is known to be the smallest for M=Cu while it is much bigger for NiFe2O4. Recently, several works on nanometer-sized ferrites obtained by milling have found that the resulting nanoparticles are structurally and magnetically disordered due to changes in the inversion degree, creation of oxygen vacancies, or amorphization of the structure. |
|
When M is a magnetic ion, the total magnetic moment of the eight blocks that form the unit cell in MFe2O4 is due to uncompensated magnetic moments of the A and/or B magnetic sublattices. For a completely inverted structure, such as CuFe2O4, the magnetic moment per unit cell is m=8´1mB=8mB, assuming that each Cu2+ ion contributes 1 mB, where mB is the Bohr magneton. |